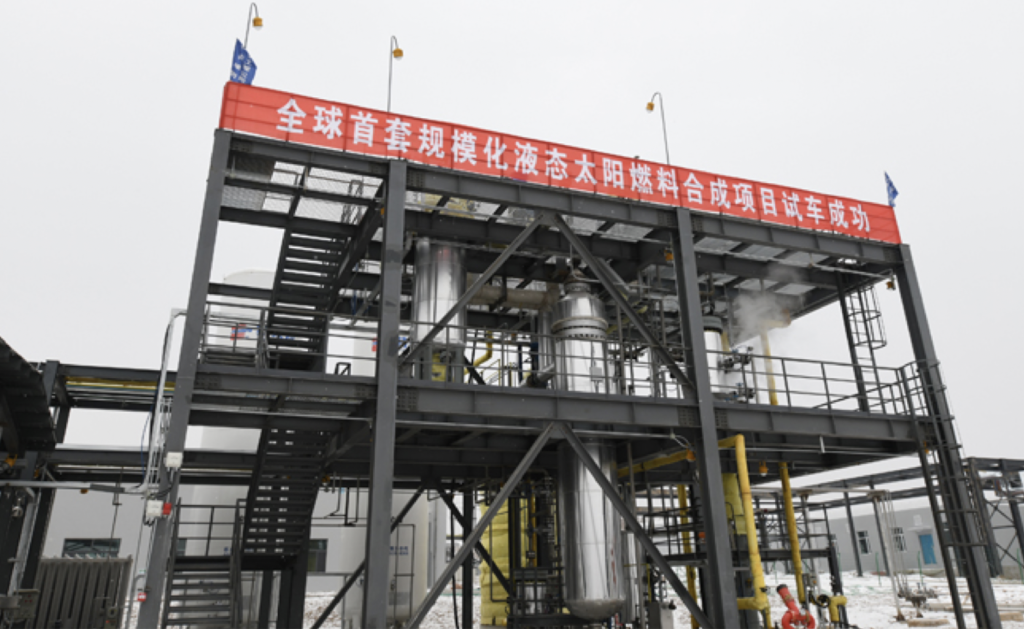
The project initiated by LI Can of CAS Dalian Institute of Chemical Physics combines hydrogen production by electrocatalytic water splitting with highly selective, carbon dioxide hydrogenation to methanol. Energy consumption is reduced to 4.0-4.2 kWh / m3 hydrogen. Carbon dioxide hydrogenation to methanol uses a bimetal oxide catalyst (ZnO-ZrO2). Methanol selectivity is greater than 90%, and degradation of the catalyst after 3000 hours of operation is less than 2%. A1,000-ton scale demonstration project was commissioned in the Green Chemical Park in Lanzhou New District.
CAS news release, January 17, 2020